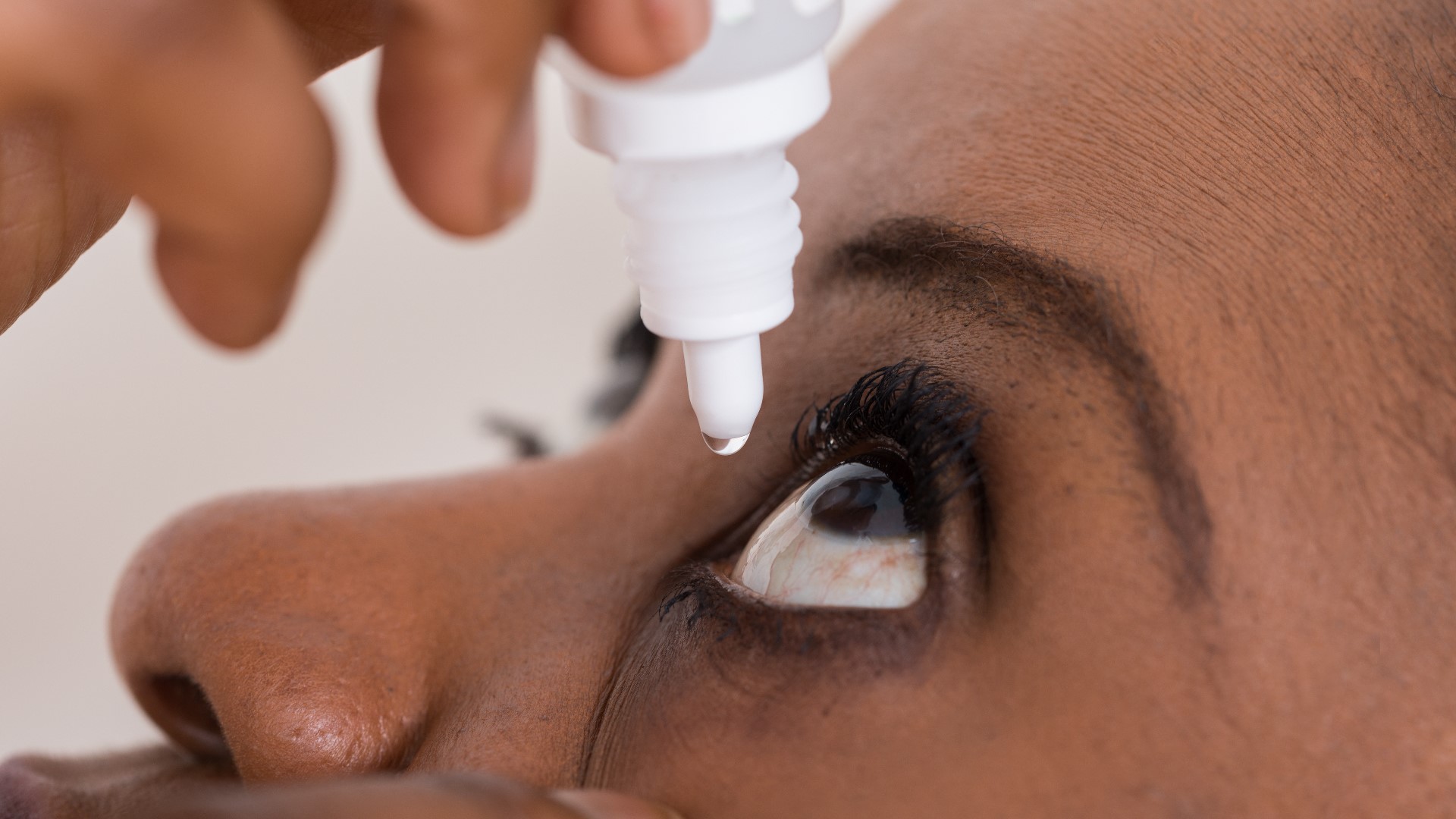
WASHINGTON — The FDA says eight companies have been making or selling unapproved eye drugs in violation of federal law, and have sent warning letters to the companies because the products could cause serious problems.
The eight companies include CVS and Walgreens, as well as six manufacturers of eye products. The full list of companies is:
The U.S. Food and Drug Administration said the companies are illegally marketing eye drops that claim to treat common conditions such as pink eye, cataracts or glaucoma. The marketing is illegal because the products aren't approved in the U.S. to treat those conditions.
Some of these products have also had quality issues related to not being sterile, the FDA said.
The companies were told by the FDA they have 15 days to respond, describing how they plan to correct the issues. If they don't, the FDA warned they will pursue legal action and ask a court to order the companies to stop manufacturing or selling the eye drops.
Part of the issue comes from the fact that the drugs are applied directly to the eyes. Drugs taken this way can bypass some of the body's natural defenses, potentially causing serious damage.
A number of the products under scrutiny list silver, silver sulfate, silver sulphate or argentum as ingredients. Long-term use of these ingredients can cause permanent changes to the body. Tissues such as those found in the skin and eyes can become gray permanently in a process called argyria.
The FDA said it was concerned that unapproved drugs claiming to treat or cure serious medical conditions such as glaucoma may keep consumers from going through with medical treatments that have been approved as safe and effective.
“The FDA is committed to ensuring the medicines Americans take are safe, effective and of high quality," said Jill Furman, director of the Office of Compliance for the FDA’s Center for Drug Evaluation and Research, in a statement announcing the warning letters. “We will continue to investigate potentially harmful eye products and work to ensure violative products stay off store shelves so that consumers can continue taking the medicines they need without concern.”
Anybody who has used an eye product included in one of the warning letters should talk to their optometrist. Any health issues related to their use can be reported to the FDA's MedWatch program.